Vermont Businesses Beware of Deceptive Business Filing Service
Contact: Charity R. Clark, Chief of Staff, 802-828-3171
Contact: Charity R. Clark, Chief of Staff, 802-828-3171
Contact: Charity R. Clark, Chief of Staff, 802-828-3171
Attorney General T.J. Donovan today expressed disappointment when reacting to the plan Purdue Pharma filed in bankruptcy court. Along with 23 other states, Attorney General Donovan released the below statement:
“We are disappointed in this plan. While it contains improvements over the proposal that Purdue announced and we rejected in September 2019, it falls short of the accountability that families and survivors deserve.
Contact: Charity R. Clark, Chief of Staff, 802-828-3171
Contact: Charity R. Clark, Chief of Staff, 802-828-3171
Contact: Charity R. Clark, Chief of Staff, 802-828-3171
MONTPELIER – Attorney General T.J. Donovan today announced that Vermont, as part of a coalition of 41 attorneys general, has settled with Retrieval-Masters Creditors Bureau, doing business as American Medical Collection Agency (“AMCA”) resolving a multistate investigation into the 2019 data breach that exposed the personal information of over 7 million individuals, including 2,889 Vermont residents, and potentially exposed the personal information of up to 21 million individuals throughout the United States.
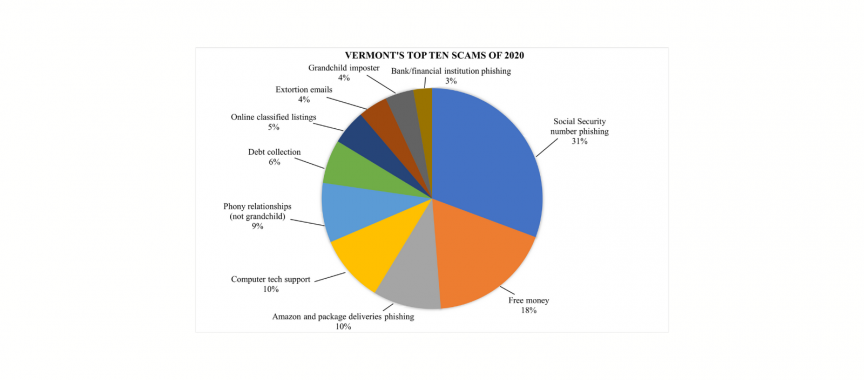
Contact: Lisa Jensen, Assistant Director of the Consumer Assistance Program, 800-649-2424.
Contact: Charity R. Clark, Chief of Staff, 802-828-3171
Contact: Charity R. Clark, Chief of Staff, 802-828-3171
“I am pleased with the Court’s ruling upholding Vermont’s ban on large capacity magazines. This is a win for common sense gun policies and for public safety.
Contact: Charity R. Clark, Chief of Staff, 802-828-3171
Contact: Charity R. Clark, Chief of Staff, 802-828-3171
Contact: Charity R. Clark, Chief of Staff, 802-828-3171
Concerned about identity theft? The best way to know that no one is using your personal information is to monitor your credit. The Attorney General’s Consumer Assistance Program (CAP) recommends that Vermonters review their credit reports now, and regularly, to make sure that no unauthorized accounts are being reported.
Contact: Charity R. Clark, Chief of Staff, 802-828-3171
Attorney General T.J. Donovan announced recent environmental actions taken by his office to protect Vermont’s environment and issued the following statement: “Vermonters should know that my team will continue to protect our environment. We also know that the climate crisis is real and the time to act is now. We are glad to again have leadership in Washington that believes in science and protecting our natural world for future generations, but our work here in Vermont will continue.”
Contact: Charity R. Clark, Chief of Staff, 802-828-3171
Contact: Doug Hoffer, Vermont State Auditor, 802-864-5711 | Charity R. Clark, Chief of Staff, 802-828-3171
Contact: Charity R. Clark, Chief of Staff, 802-595-8679
Attorney General T.J. Donovan wants to hear from young Vermonters about their thoughts on the environment. For the first time ever, the Attorney General’s Office is holding an Earth Day Essay Challenge and asking 5th and 6th graders all around Vermont to submit essays on the environment.
Contact: Charity R. Clark, Chief of Staff, 802-828-3171
The Attorney General’s Office announced that Aita Gurung today stipulated to his competency in Chittenden Superior Court, Criminal Division, the Honorable Samuel Hoar, Jr. presiding. Mr. Gurung is accused of Murder in the First Degree for the killing of his wife, Yogeswari Khadka, and Attempted Murder in the First Degree for the near-fatal attack on his mother-in-law, Tulasa Rimal. Today’s stipulation resolves the issue of Mr. Gurung’s competency and allows the State to move forward with its prosecution.
Contact: Charity R. Clark, Chief of Staff, 802-828-3171
Contact: Charity R. Clark, Chief of Staff, 802-828-3171
Contact: Charity R. Clark, Chief of Staff, 802-828-3171
Contact: Charity R. Clark, Chief of Staff, 802-828-3171
Attorney General T.J. Donovan today requested that Acting U.S. Attorney General Jeffrey A. Rosen commence a criminal investigation into the activities of President Donald Trump and rioters in relation to the events occurring at the U.S. Capitol yesterday. Attorney General Donovan wrote in the letter to Acting Attorney General Rosen:
Contact: Charity R. Clark, Chief of Staff, 802-828-3171
Contact: Charity R. Clark, Chief of Staff, 802-828-3171
Contact: Charity R. Clark, Chief of Staff, 802-828-3171
Attorney General T.J. Donovan, along with the attorneys general of 27 states, has entered into a settlement with Sabre Corporation that resolves an investigation into the 2017 data breach of Sabre Hospitality Solutions’ hotel booking system. The breach exposed the data of approximately 1.3 million credit cards. The settlement requires a payment of $2.4 million, of which the State of Vermont will receive $397,000 and injunctive relief.
Contact: Charity R. Clark, Chief of Staff, 802-828-3171
Attorney General T.J. Donovan announced today that Jason King, 42, and William Smith, 54, both of St. Albans, Vermont, were arraigned in Vermont Superior Court, Franklin Division, on December 18, 2020 for child sexual exploitation related charges.
Contact: Charity R. Clark, Chief of Staff, 802-828-3171
Contact: Charity R. Clark, Chief of Staff, 802-828-3171
Contact: Charity R. Clark, Chief of Staff, 802-828-3171
Contact: Charity R. Clark, Chief of Staff, 802-828-3171
Contact: Charity R. Clark, Chief of Staff, 802-828-3171
Contact: Charity R. Clark, Chief of Staff, 802-828-3171
Bipartisan Coalition of 48 Attorneys General Charge Anticompetitive Conduct: Facebook Thwarted Competition, Reduced Consumer Privacy for Profits
CONTACT: Charity R. Clark, Chief of Staff, 802-828-3171
Molly Dillon, Deputy Commissioner of Banking, Dept. of Financial Regulation, 802-828-4874
Seventy-Eight Vermonters to Receive $100,000 in Restitution
CONTACT: Charity R. Clark, Chief of Staff, 802-828-3171
Use of force analyzed under both current Vermont law and new statute
CONTACT: Charity R. Clark, Chief of Staff, 802-828-3171
Contact: Charity R. Clark, Chief of Staff, 802-828-3171
CONTACT: Jamie Renner, Assistant Attorney General, 802-828-3171
New Guide Lays out Help Options for Older Vermonters Experiencing Abuse or Exploitation
Contact: Charity R. Clark, Chief of Staff, 802-828-3171
Following President Donald Trump falsely declaring victory in the presidential election, Attorney General T.J. Donovan is reassuring Vermonters that he will protect the rule of law by ensuring that every vote is counted.
“If democracy is going to work, we must respect the will of the people and that means we count every legal vote,” said Attorney General Donovan. “Voters will determine the outcome of the election.”
U.S. Department of Labor Rule Would Strip Workers of Key Protections
Contact: Charity R. Clark, Chief of Staff, 802-828-3171
Contact: Charity R. Clark, Chief of Staff, 802-828-3171
Contact: Philip Back, Assistant Attorney General, 802-828-3171
The Vermont Democratic Party and the Burlington Democratic Committee will pay a penalty to the State of Vermont for campaign finance reporting violations. According to a settlement reached today, the Vermont Democratic Party and the Burlington Democratic Committee failed to timely file reports regarding the March 3, 2020 Town Meeting Day election, in violation of 17 V.S.A. § 2964(b)(1), for which they will collectively pay $2750 as a civil penalty.
Contact: Charity R. Clark, Chief of Staff, 802-828-3171
A federal judge has ruled in favor of the lawsuit filed earlier this year by Attorney General T.J. Donovan to protect food assistance.
Contact: Charity R. Clark, Chief of Staff, 802-828-3171
Today, Jayveon Caballero was sentenced to serve 25 years to life for the murder of Markus Austin in Montpelier, Vermont. Mr. Caballero was convicted of Murder in the Second Degree after a jury trial in November 2019. The case was heard by Judge Mary Morrissey in Washington Superior Court, Criminal Division. Assistant Attorneys General Elizabeth Anderson and John Waszak prosecuted the case on behalf of the State of Vermont. In light of today’s sentencing, Attorney General T.J. Donovan issues the following statement:
Contact: Charity R. Clark, Chief of Staff, 802-828-3171
Contact: Charity R. Clark, Chief of Staff, 802-828-3171
Contact: Charity R. Clark, Chief of Staff, 802-828-3171
Contact: Charity R. Clark, Chief of Staff, 802-828-3171
Contact: Charity R. Clark, Chief of Staff, 802-828-3171